Research Summary
Colloidal nanocrystals are powerful models for probing the structure and reactivity of materials in solution. They can be synthesized with a size and shape distribution of only a few atoms and in this sense are nearly molecular in nature. Our group is especially interested in colloidal semiconductor quantum dots that are the key light emitters and absorbers in cutting edge luminescent displays and lighting devices. Understanding the underlying chemistry of their surfaces and the mechanisms of charge recombination are the keys to saving vast quantities of energy, particularly in lighting applications, a utility that consumes > 5% of global power production.
The outstanding monodispersity common to modern samples is the surprising result of complex, multi-stage crystal growth mechanisms that span many length and time scales. We are studying how such seemingly complicated reactions, can lead to such well-defined products. In addition, we use the materials to study the interactions of ligands with surfaces. The structure of surfaces is key to the reactivity of catalysts and the recombination of photoexcited charges in semiconductors. Colloidal nanocrystals provide a powerful tool with which to probe these issues using solution techniques, including NMR spectroscopy. This is a rapidly growing branch physical organic and organometallic chemistry that will greatly accelerate the design and manipulation of new materials.
Mechanistic Studies of Crystal Growth

Modern colloid chemistry can control the size and shape of nanometer scale crystals to the precision of a few atoms. This has largely been achieved empirically rather than by designing methods based on sound mechanistic understanding. We aim to establish a foundational understanding of precursor conversion, nucleation, and growth of these crystals, especially in III-V, II-VI, and IV-VI semiconductors.
Classical Nucleation Theory and the qualitative La Mer picture of nucleation is typically invoked to explain the formation of semiconductor nanocrystals in colloidal solution, although the relevance of these models is increasingly under scrutiny. Our research is showing how these “one size fits all” thermodynamic models of nucleation fail to capture the specific local structure and chemical bonding of the metastable and pre-nucleation structures that differentiate various materials systems.

To study crystal growth we utilize several in-situ spectroscopic probes including multinuclear nuclear magenetic resonance (NMR), UV-vis/NIR absorbance and photluminescence spectroscopies, as well as small angle X-ray scattering, and pair distribution function analysis (see Figure). These studies are made possible by multiple collaborations with national X-Ray synchrotron facilities including NSLS-II at Brookhaven National Lab, Argonne National Lab, European Synchrotron Radiation Facility as well as automated high throughput synthesis robotics at the Molecular Foundry at Lawrence Berkeley National Lab. Experimental results are evaluated theoretically by our collaborators, including Prof. Baron Peters Group at the University of Illinois at Urbana Champaign.
- Quantum Dot Synthesis and Solid State Lighting
By studying the mechanism of precursor reactions, we aim to relate the kinetics of solute supply to the outcome of crystallization. We have found that the rate at which precursors are converted to monomers determines the number of nuclei and the steady state supersaturation during growth. These parameters are key to controlling nanocrystal shape, size, dispersity, and composition. In this vein, we have developed libraries of substituted chalcogenocarbonyl and aminophosphines for conversion to metal chalcogenide and indium phosphide nanostructures, respectively. In reactions with lead carboxylates, our libraries of thio- and selenoureas allow access to a wide size range of PbS and PbSe nanocrystals whose distributions approach the homogenous limit. With our unique ability to tune the reactivity of precursors orthogonal to crystallization, we have probed the underlying mechanisms of nanocrystal nucleation and growth in metal chalcogenides and phosphides.

Chalcogenoureas Give Highly Monodisperse PbS and PbSe
In reactions with lead carboxylates, our libraries of thio- and selenoureas allow access to a wide size range of PbS and PbSe nanocrystals whose distributions approach the homogenous limit. By manipulating the substitution pattern about the chalcogenourea, conversion reactivity towards PbSe and PbS can be tuned by four and five orders of magnitude, respectively. For both systems we find the rate of precursor conversion correlates with the extent of crystal nucleation and therefore serves as an effective method for tuning final nanocrystal size. In the case of PbSe we find that line widths are dominated by intrinsic single particle spectral broadening, as observed using spectral hole burning measurements. Here we were able to elucidate that intrinsic broadening decreases with increasing size due to exciton-phonon coupling rather than broadening caused by the size distribution.

Quantum Dots for Solid State Lighting
Leveraging our tunable library of chalcogenocarbonyl precursors, we have devised synthetically convenient methods to tune the microstructure of metal chalcogenide heterostructures. Specifically, manipulations in the reactivity of thio- and selenoureas with cadmium carboxylates allow for the one-pot synthesis of core-shell (CdSe/CdS and CdS/CdSe) and solid solution (CdSxSe1-x) heterostructures. Utilizing our unique synthetic platform and a high throughput screening method at the Lawrence Berkeley National Lab (WANDA), we have identified structures relevant for energy efficient, warm white, solid state lighting. Industrial testing of our materials in LED settings has displayed performance competitive with industry standards. Such tests have emphasized the importance of protective shell layers in the long-term performance and stability of the nanocrystals. These concerns are being addressed by ongoing efforts to develop synthetically controlled methods of zinc chalcogenide shell deposition. Furthermore, exercising our fundamental understanding of InP nanostructures, we are actively targeting cadmium-free material systems for solid state lighting applications.

Mechanism of InP Nanocrystal Formation
Using our aminophosphine library, we have arrived at the surprising conclusion that monodisperse InP nanocrystals can form under conditions where nucleation continues throughout the synthesis. This finding starkly contrasts the widely believed theory that narrow size distributions require a “burst” of nucleation to occur at early reaction times and separate from growth. We measure the length of the nucleation period and show that this does not explain the size distribution. We therefore conclude that size distributions are controlled by size dependent growth kinetics, which is a stark counterpoint to the La Mer hypothesis and opens a new chapter in the theory of size and size distribution control during crystal growth. Beyond novel mechanistic insights, our new library displays, for the first time, precise size control of III-V nanocrystals by tuning of the kinetics of solute supply.
Selected Publications
"Continuous Nucleation and Size-Dependent Growth of Indium Phosphide Nanocrystals"
Brandon M. McMurtry, ..., Jonathan S. Owen Chem. Mater., 2020, 32, 4358–4368.
“Precursor reaction kinetics control compositional grading and size of CdSe1−xSx nanocrystal heterostructures.”
Leslie S. Hamachi, ..., Jonathan S. Owen. Chem. Sci., 2019, 10, 6539-6552.
“A tunable library of selenourea precursors to PbSe nanocrystals with size distributions near the homogeneous limit.”
Michael P. Campos, ..., Jonathan S. Owen. J. Am. Chem. Soc., 2017, 139, 2296-2305.
“A tunable library of substituted thiourea precursors to metal sulfide nanocrystals.”
Mark P. Hendricks, ..., Jonathan S. Owen, Science, 2015, 348, 1226-1230.
Surface Coordination Chemistry
The performance and stability of nanoparticles is largely governed by interactions between particle surfaces and the surrounding media. Control over properties like photoluminescence quantum yield and photochemical robustness is essential in incorporating quantum dots into LEDs and solid-state lighting technologies. We have a long-standing interest in understanding nanocrystal surface structure, its influence on electronic structure and optical performance, and the mechanisms through which ligands are exchanged.

Metal-carboxylate Ligand Exchange
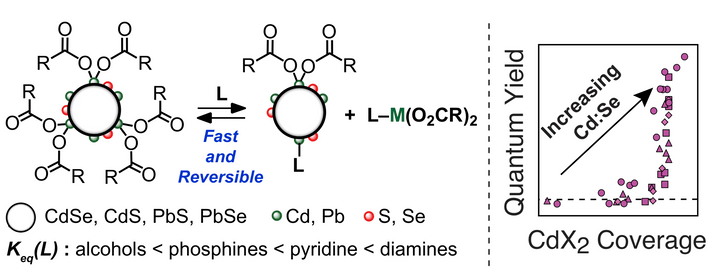
Metal-carboxylate complexes (MX2, M = Pb, Cd, X = carboxylate) on nanocrystal surfaces can be understood as Z-type ligands. We have observed the facile displacement of surface MX2 ligands by L-type Lewis bases using NMR spectroscopy. These results reveal that Pb and Cd-based nanocrystal surfaces are dynamic, and that their chemical formulas depend on solution concentration and composition. Moreover, this observed L-promoted Z-ligand displacement has important consequences for the optical properties of metal chalcogenide nanocrystals; photoluminescence quantum yield varies by over one order of magnitude in CdS and CdSe nanocrystals depending on the extent of ligand exchange.

Ion Pairing and Colloidal Dispersions
We have prepared rare examples of CdSe nanocrystals with surfaces bound exclusively by neutral L-type ligands (such as primary amines, TOPO, 4-ethylpyridine, and Bu3P). We found that the resulting nanocrystals are unstable toward aggregation except in the presence of moderate (>0.1 M) L-ligand concentrations. These results demonstrate that prevalent neutral donors are in fact insufficient to stabilize colloidal nanocrystals. Instead, we found the presence of trace acidic impurities—for example, carboxylic acids—leads to ion pair surfactants that tightly bind the nanocrystal surface.

Stereoelectronic Factors that Control Ligand Binding
Building on our ability to prepare nanocrystals coordinated by L-type ligands, we have conducted a competitive binding investigation to understand the stereoelectronic factors that dictate ligand binding at nanocrystal surfaces. We find that ligand sterics are dominant in determining binding affinity; interesting, this means that stronger Lewis bases like PBu3 are displaced by weaker Lewis bases with a smaller steric profile such as pyridine.
Selected Publications
"Stereoelectronic Effects on the Binding of Neutral Lewis Bases to CdSe Nanocrystals."
Nicholas C. Anderson, ..., Jonathan S. Owen. J. Am. Chem. Soc., 2018, 140, 7199.
"Tight Binding of Carboxylate, Phosphonate, and Carbamate Anions to Stoichiometric CdSe Nanocrystals."
Peter E. Chen, ..., Jonathan S. Owen. J. Am. Chem. Soc., 2017, 139, 3227.
“Ligand Exchange and the Stoichiometry of Metal Chalcogenide Nanocrystals: Spectroscopic Observation of Facile Metal-Carboxylate Displacement and Binding.”
Nicholas C. Anderson, ..., Jonathan S. Owen. J. Am. Chem. Soc., 2013, 135, 18536.
Contact Us
Lab: 212-854-4686
501 Havemeyer Hall
© 2019